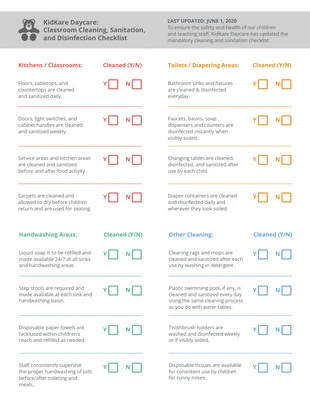
Data and Safety Monitoring Plan Checklist Template
This data safety monitoring report template safeguards the measures to be taken during data collection and documentation.
100% customizable templates
Millions of photos, icons, charts and graphics
AI-powered editing features
Effortlessly share, download, embed and publish
Easily generate QR codes for your designs
- SizeLetter (8.5 x 11 in)
- File typePNG, PDF, PowerPoint
- Planpremium
A data and safety monitoring plan, or DSMP, is a quality assurance plan for a research study. It prospectively identifies and documents monitoring activities intended to protect the subjects' safety, the data's validity, and the research study's integrity. The DSMP may also determine when to terminate a subject's participation (i.e., individual stopping rules) and the appropriate termination of the study (i.e., study stopping rules). DSMPs are important for clinical investigations because they help ensure that subjects are safe and that the data collected is accurate and reliable. By establishing a system for appropriate oversight and attention to protecting human subjects, the investigator, research team, and independent reviewer can ensure that researchers will conduct the clinical investigation with integrity. When developing a data and safety monitoring plan, there are a few key things to consider. First, it is essential to identify the risks associated with the study. You can do this by assessing the study's nature, size, and complexity. Then, it is necessary to create a monitoring strategy to help avoid or minimize these risks.